Abstract
Objective. The effects and perception of aging are directly reflected in the health and condition of the skin. Beauty and antiaging products largely focus on treatment of the skin with an outside-in strategy. There is demand for “beauty from within” products that support underlying internal processes necessary for healthy and vital skin. This study assesses the effectiveness of methylsulfonylmethane (MSM) as an oral supplement on skin health using expert grading, instrumental measurements, and participant self-evaluation. Methods. An initial preclinical in vitro gene marker study evaluated the effects of 2.5% MSM solution on the expression of 92 genes associated with skin function. The primary double-blind, placebo-controlled clinical trial randomized 20 female participants to receive either 3 g per day of MSM or placebo over 16 weeks. Skin health was evaluated through expert grading, instrumentation, and participant self-assessment at weeks 8 and 16. Results. MSM regulates the genomic expression of key genes responsible for skin health and the prevention of aging. Furthermore, MSM supplementation showed statistically significant improvements over placebo by expert grading in crow’s feet and skin firmness, and statistically significant improvements from baseline in crow’s feet, skin firmness, tone, and texture. Using photo instrumentation analysis, MSM supplementation produced statistically significant improvements over placebo for wrinkle (crow’s feet) total count, length, severity, and deep line counts and for wrinkles (global) total count, length, and severity. The product was well tolerated, and overall, the MSM group gave more favorable self-assessment than the placebo group, though the improvement was not statistically significant. Conclusion. MSM supplementation appears to benefit skin health, primarily the reduction of fine lines and wrinkles. Effects on gene expression may partially account for the benefits, but further research is needed to verify results and mechanism of action.
Introduction
The skin is the largest organ of the body and serves several purposes in the lives of individuals. Functionally, the skin provides a barrier from exogenous factors, maintains body temperature, and prevents water loss. Socially and psychologically, the skin acts as an expression of aging and a determinant of physical appeal.1 Skin health is inextricably linked to both physical health outcomes like infection and hydration and to psychological outcomes like anxiety and depression.2,3 Skin firmness and the size and number of wrinkles are strongly associated with the perception of age, making skin the focus of many antiaging treatments.4 Hereditary factors only account for approximately 3% of aging, and the skin is especially vulnerable to aging damage from both endogenous and exogenous factors.5 Rapid cell production and turnover requires consistent and sufficient nutrients for healthy growth and function. Environmental factors like toxins, free radicals, humidity, and temperature can damage the structure and function of skin thus affecting its barrier properties. Internal factors like stress, malnourishment, and inflammation can impair the skin’s growth, maintenance, and ability to combat external factors.6-8
Traditionally, the approach to skin has been from the outside-in, through topical application and direct treatments. Recently, there has been a strong interest in an inside-out approach using nutrition and nutraceuticals to support skin function.9 There is a demand for “beauty from within” products that provide support endogenously to reduce the effects of aging and damage. Termed nutricosmetics, these products currently include antioxidants like polyphenols, phytonutrients, and vitamins C and E, as well as structural components like hydrolyzed collagen and hyaluronan.10 Methylsulfonylmethane (MSM) is an organosulfur found in a variety of foods including milk, grains, fruits, and vegetables.11Also known as dimethyl sulfone, it is associated with a variety of health claims including relief of pain associated with osteoarthritis, allergic rhinitis, and inflammation.12-14 Previous MSM research indicates that it may be a good candidate as a nutricosmetic by providing organic sulfur, reducing inflammation, and supporting the body’s intrinsic antioxidant pathways. Sulfur has long been associated with skin health because of its fundamental role in physiological processes, including the synthesis of collagen, hyaluronic acid, and keratohyalin—the most abundant matrix molecules in the skin.15-17 There is evidence that MSM sulfur can be incorporated into the sulfur-containing amino acids methionine and cysteine to provide a source of dietary sulfur and MSM may affect the compartmentalization and metabolism of sulfur.18 Inclusion of MSM in the sulfur pathways may provide, or possibly spare, sulfur-based components necessary for the production and maintenance fundamental structures by preventing their recycling and/or turnover rate. Overactive inflammatory responses and increased inflammation can damage dermal cells and degrade structural matrices, leading to signs of aging.19 The antiinflammatory properties of MSM may support skin health by decreasing damage done through inflammatory cascades. Studies have shown that MSM can reduce the production of interleukin (IL)-1, IL-6, tumor necrosis factor-α (TNF-α), nitric oxide (NO), prostaglandin E2 (PGE2), and nuclear factor (NF)-κB.20,21 Additionally, oxidative stress is a major contributor to cellular aging and is especially problematic in the skin, which is bombarded by free radicals from both endogenous and exogenous sources.22 MSM supports the body’s natural antioxidant pathways through increased levels of glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT).23 MSM has been shown to reduce levels of homocysteine, a molecule with damaging effects on collagen crosslinking.24
This study was conducted in 2 steps. A preclinical evaluation of the effects of MSM on gene expression in an artificial 3-dimensional (3D) skin model conducted to inform the viability and direction of the clinical phase. The purpose of the clinical evaluation was to examine the effects of oral MSM supplementation on skin health and appearance of aging. We hypothesized that supplementation of MSM at 3 g per day would decrease visible wrinkles, improve measurable skin function, and benefit participant self-assessment.
Materials/Methods
Preclinical gene marker study
This portion of the study was performed by GeneMarkers, Kalamazoo, Michigan. In the study, 2.5% OptiMSM® vs sterile water control was used to treat 3D skin cultures. Tissues were collected in the cell storage regeant RNAlater (Qiagen, Venlo, the Netherlands) for gene expression analysis. Gene expression was analyzed using validated TaqMan gene expression assays in the TaqMan low density array (TLDA) format. Analysis was carried out using the GeneMarkers Standard Skin Panel, which contains assays for 92 target genes and 4 endogenous control genes. RNA was isolated from EFT-400 cultures using a Qiagen RNAeasy isolation kit according to the manufacturer’s instructions for fibrous tissues. RNA concentration and purity were determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Lafayette, Colorado). High-quality RNA was isolated from all tissue samples, and cDNA was generated for each sample using a High-capacity cDNA Reverse Transcription Kit (Applied Biosystems, Thermo Scientific, Lafayette, Colorado) according to the manufacturer’s instructions. PCR reactions were run using a Life Technologies (Thermo Scientific, Lafayette, Colorado) QuantStudio 12K Flex instrument and Life Technologies validated gene expression assays in a TLDA format. Each gene was assayed in duplicate. Raw data generated with the QuantStudio 12K Flex software was imported into RealTime StatMiner software (version 4.2, Integromics, Granada, Spain) for statistical analysis using the relative quantitation (RQ) method. In the first step of an RQ analysis, the cycle threshold (CT) value of the target gene is normalized to the CT value of an endogenous control gene for each sample to generate the delta CT (dCT). The dCT values are calculated in order to normalize/control for variability between the samples that may occur during the experimental procedures. Three algorithms were used to determine the most stable endogenous control gene. Based on the stability scores of the 3 algorithms (the coefficient of variability, normfinder algorithm, and minvarmed), glucuronidase-β (GUSB) was selected as the most stable endogenous control gene. Statistics (tests) were carried out using dCT values normalized to GUSB. Statistically significant changes in gene expression (P<0.05, N=4) are reported.
Clinical Oral Study
Participants
For this portion of the study, a third-party clinical site (International Research Services Inc, Port Chester, New York) was contracted to perform this investigation. This study was conducted according to International Research Services Inc (IRSI) research policies and standard operating procedures and US and international standards of good clinical practice (US Food and Drug Administration and International Conference on Harmonisation [ICH] guidelines) and was approved by an independent institutional review board (IRB). Informed consent from each participant was obtained prior to enrollment. Twenty-three females in good health were enrolled, and 20 completed study participation (Table 1). One participant dropped out due to an adverse event, and 2 others were lost to follow-up. Inclusion criteria consisted of Caucasian females between the ages of 35 and 59 years at time of enrollment with Fitzpatrick skin type scores ranging from II to V. They were included if they had the presence of facial lines/wrinkles, dryness, roughness, and redness, as determined by an expert visual grader: lines/wrinkles score of >3 on 10-cm visual analogue scale (VAS) for both crow’s feet area and global-face; texture/smoothness of score of >2 on 10-cm VAS; dryness score of >1 on a 5-point ordinal scale; and erythema score of >1 on a 5-point ordinal scale (due to irritation). The study included only Caucasians to control for well-established variations in the ways skin responds to stress, inflammation, and environmental exposure between ethnic groups.25-27
Experimental design
Participants in this 16-week double-blind, placebo-controlled pilot study were randomized to either MSM or placebo (Table 1) using a prepared randomization code. A 1-week washout period was implemented and included the use of a daily wash, morning and evening with Camay soap (Procter & Gamble, Cincinnati, Ohio). Product use during the study was limited to the use of a daily wash, morning and evening with Cetaphil Gentle Facial Cleanser (Galderma Laboratories, LP, Fort Worth, Texas) and application of a moisturizer containing sunscreen to the face every morning as needed, with reapplication prior to sun exposure at the participant’s discretion. The treatment group took 3 g per day of OptiMSM® 1000-mg vegetarian capsules (Bergstrom Nutrition, Vancouver, Washington). The placebo group took 3 capsules each day of rice flour (matched for appearance and size). Participants, study personnel, and expert graders were blinded to treatment group. Study personnel were unblinded following statistical analysis.
Table 1. Demographics of Study Participants
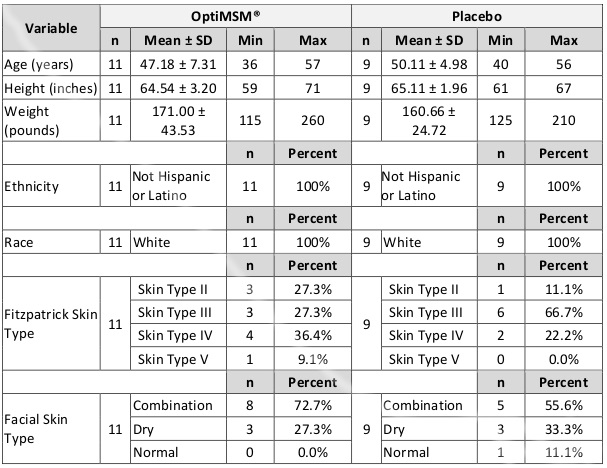
Abbreviation: SD, standard deviation.
Outcome Measures
Expert analysis, instrumental measurements, and subjective questionnaires were collected at baseline, week 8, and week 16.
Expert analysis of participant skin was collected at each time point and graded for wrinkles (crow’s feet and global), tone, texture/smoothness, elasticity, and firmness using a 10-cm VAS. For wrinkle analysis, the VAS ranged from “None” (0 cm) to, “Numerous lines/wrinkles. Severe, deep” (10 cm). For tone analysis, the VAS ranged from “Even, healthy skin color” (0 cm) to “Uneven, discolored appearance” (10 cm). For texture/smoothness analysis, the VAS ranged from “Smooth, silky appearance” (0 cm) to “Coarse, rough appearance” (10 cm). For firmness analysis, the VAS ranged from “Firm, tight with good recoil properties” (0 cm) to “Loose with poor recoil properties” (10 cm). Measurements were made in cm to the nearest 0.1 cm.
Digital photographs were taken of the front and left side of the participants’ faces using a Clarity™ 2D Research Ti camera (18 megapixel single-lens reflex camera; BrighTex Bio-Photonics, San Jose, California) under standard light with a matte black background and cape for analysis of facial lines/wrinkles and crow’s feet. Multispectral lighting reveals skin conditions on and beneath the skin’s surface layer, and the system uses skin feature recognition to apply automated skin segmentation and zone mapping for subsequent analysis.
A cutometer was used to measure viscoelastic properties by applying suction to the skin surface and measuring penetration depth. A corneometer was used to measure skin moisture by measuring electrical capacitance through 2 closely spaced electrodes. A vapometer was used to measure skin barrier function by measuring transepidermal water loss (TEWL). TEWL is calculated by measuring relative humidity and temperature in a closed cylindrical chamber on the skin surface. A spectrophotometric intracutaneous analysis scope (SIAscope, Astron Clinica Limited, Toft, United Kingdom) was used to analyze melanin, hemoglobin, and collagen by imaging the skin with high-intensity light-emitting diodes at discreet wavelengths.
Subjective questionnaires were taken at each timepoint to measure participant self-perception of skin qualities, and tolerance of product was monitored throughout the study. The questionnaires were split into 2 parts: the first part asked the participant’s agreement level on a 3-point scale with a given statement regarding the product, and the second asked about percentage of improvement as perceived by the participant (0-100%).
Statistical analysis
Demographics, visual and instrumental assessments, and tolerance were analyzed with descriptive statistics, paired t-test (monadic), and unpaired t-tests. Subjective questionnaires were analyzed with descriptive statistics and Wilcoxon rank sum tests. Statistical significance was set at P<0.05, and a statistical trend was set at P<0.01.
Results
Preclinical gene marker study
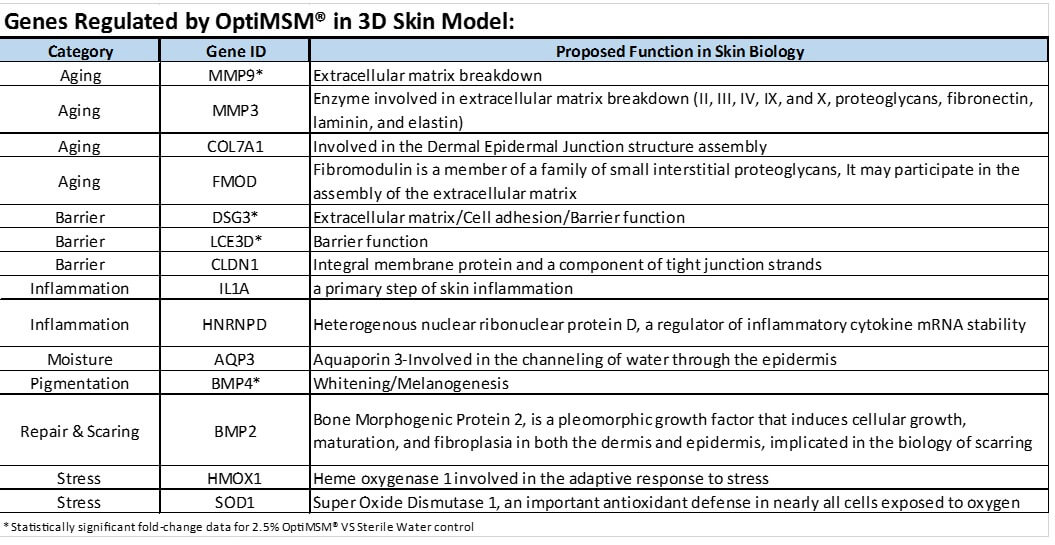
It was demonstrated that MSM influenced skin on a genetic level, and it seems apparent that MSM works in part by regulating a select number of genes responsible for inflammation, skin barrier, and moisturization, as well as those genes involved in the structural integrity of the skin which are associated with the aging process (Table 2).
Table 2. Genes Modulated by Methylsulfonylmethane in 3-dimesional Skin Equivalents
Clinical Oral Study
Tolerance
Comparative data analysis revealed no statistically significant performance differences between results from groups using MSM and placebo. One adverse event was reported from a participant in the placebo group, and she chose to discontinue the study.
Expert visual grading
When expert visual grading results were examined for MSM the data revealed a statistically significant improvement from mean baseline at week 8 (‒0.31 cm±0.39, P=0.024) and week 16 (‒0.84 cm±0.67, P=0.002) for crow’s feet lines/wrinkles; tone at week 8 (‒0.45 cm±0.60, P=0.032) and week 16 (‒0.70 cm±0.93 P=0.031); texture/smoothness at week 8 (‒0.80 cm±1.13, P=0.040) and week 16 (‒1.50 cm±0.94, P<0.001); and improvements at week 16 for elasticity (‒0.53 cm±0.53, P=0.007) and firmness (‒0.53 cm±0.47, P=0.004). Results for placebo revealed statistically significant worsening for skin firmness at week 8 (data not shown). Comparison analysis revealed statistically significant performance differences between MSM and placebo for firmness at week 8 (P=0.046) and a statistical trend with regard to crow’s feet lines/wrinkles at weeks 8 and 16 (P=0.063, P=0.090; Figure 1).
Figure 1. Expert clinical grader evaluation compared to baseline: crow’s feet line/wrinkles (1A) and firmness (1B). Compared to baseline crow’s feet line/wrinkles (1C), firmness (1D), tone (1E), and skin texture (1F).
1A
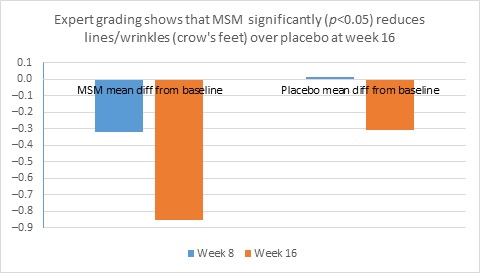
1B
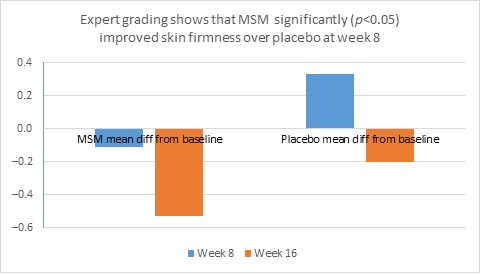
1C
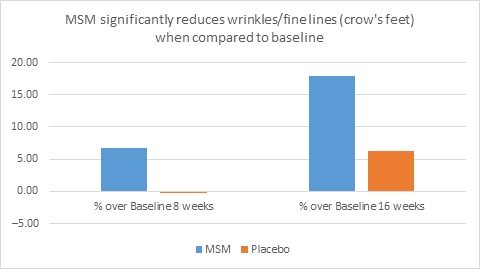
1D
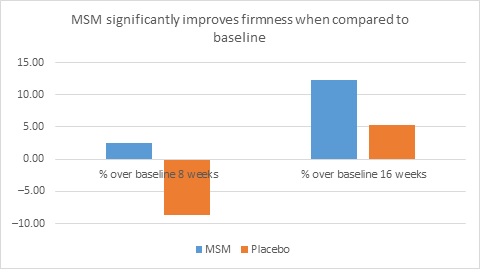
1E
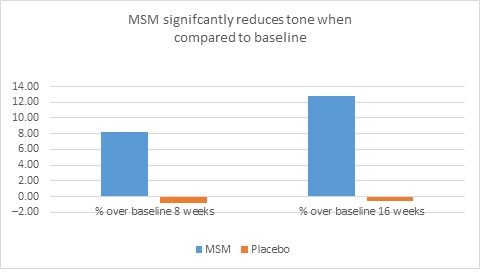
1F
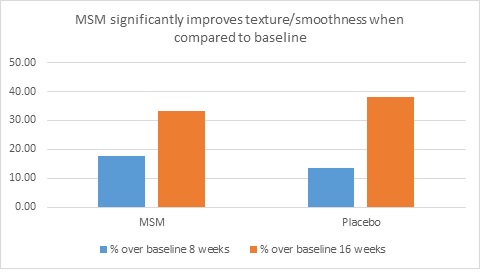
Instrumental Assessments
Vapometer results for the MSM group showed statistically significant improvements when compared to baseline values for skin barrier function at week 8 (‒4.72±6.52, P=0.037) and week 16 (‒5.79±5.54, P=0.006), with 100% of participants in the MSM group showing improvement at week 16, but these results were not statistically significant compared to placebo (data not shown). Cutometer results for skin firmness and elasticity did not show any statistically significant improvements over baseline (data not shown). SIAscope results showed statistically significant hemoglobin improvements for MSM when compared to baseline at week 8 (15.69±19.02, P=0.021) and week 16 (14.10±19.68, P=0.039) but not when compared to placebo. A statistically significant increase in melanin was observed in the MSM group at week 16 when compared to baseline (14.74±20.72, P=0.040) but was not statistically significant when compared to placebo (data not shown).
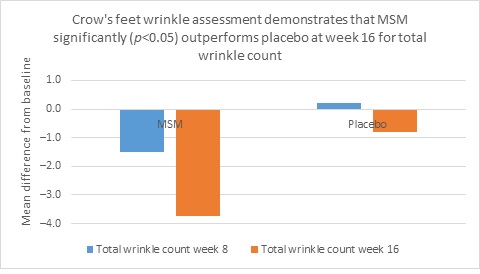
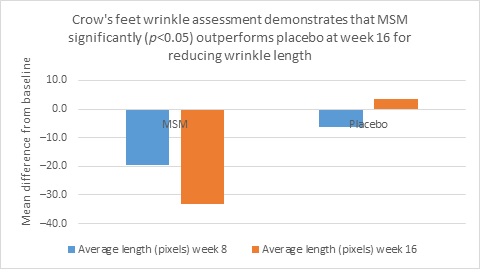
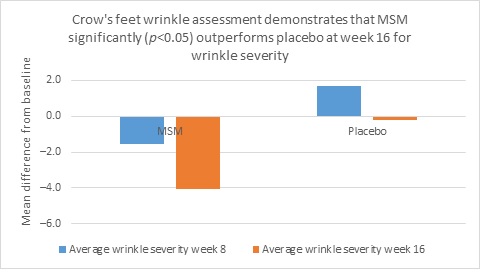
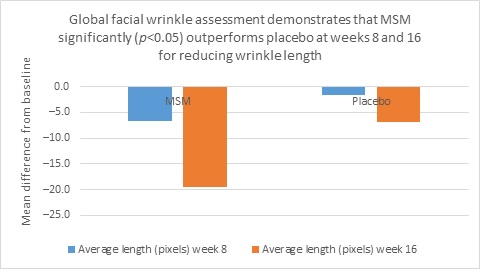
Clarity™ Pro data, used to analyze wrinkle parameters digitally, shows that in all but 1 instance, mean results for the participants using MSM were better than those for participants using placebo and improvements over placebo reached statistical significance in 7 categories. Results for analysis of crow’s feet fine lines and wrinkles showed MSM reduced total wrinkle count by 2.95 over placebo at week 16 (P=0.012; Figure 2A); reduced average length by 36.74 pixels over placebo at week 16 (P=0.019; Figure 2B); reduced average wrinkle severity by 3.82 over placebo at week 16 (P=0.024; Figure 2C); and reduced deep line counts by 0.78 over placebo at week 16 (P=0.036; Figure 2D). Results for analysis of global lines and wrinkles showed MSM reduced total wrinkle count by 10.20 wrinkles over placebo at week 16 (P=0.042; Figure 3A); reduced the average length by 5.10 pixels at week 8 and 12.60 pixels at week 16 over placebo (P=0.032, P=0.004; Figure 3B); and reduced wrinkle severity by 8.44 over placebo at week 16 (P<0.001; Figure 3C). A representative example of Clarity™ Pro analysis using digital lines can be seen in Figure 4.
Figure 2. Clarity™ Pro image analysis (pixel count) of fine lines and wrinkles (crow’s feet): total wrinkles (2A), wrinkle length (2B), wrinkle severity (2C), and deep line count (2D).
2A
2B
2C
2D
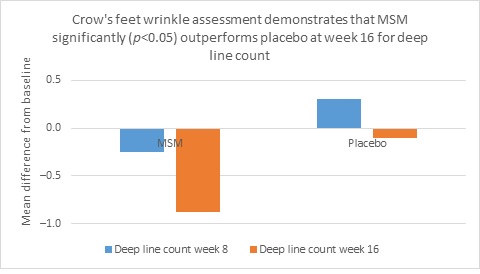
Figure 3. Clarity™ Pro image analysis (pixel count) of fine lines and wrinkles (global): total wrinkles (3A), wrinkle length (3B), and wrinkle severity (3C).
3A

3B
3C
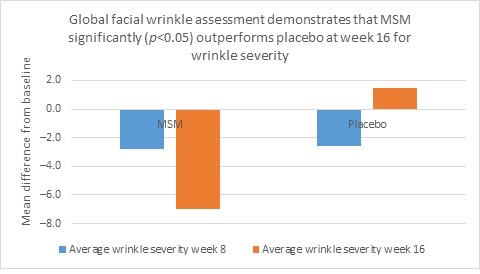
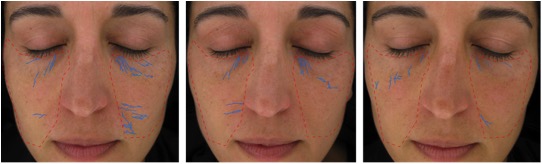
Figure 4. A representative example of Clarity™ Pro imaging shows the computer generated wrinkle tracings of the left side for crow’s feet analysis (4A) and frontal view for global assessment (4B) for a participant in the methylsulfonylmethane group.
Figure 4A. From left to right: baseline, week 8, week 16 (Participant 1)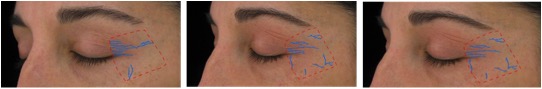
Figure 4B. From left to right: baseline, week 8, week 16 (Participant 1)
Subjective assessment
These data were further supported by self-assessment data collected from the participants at week 16 from both groups. Participants taking MSM had more favorable self-assessment scores than those taking the placebo, based on the percentage of participants responding favorably (in excess of 50% for each question posed and choosing the top 2 scores relating to improvement on the scale of 1 to 5; Figure 5). Although statistical significance was not reached for any individual question, the global assessment is an important demonstration of congruence between the expert grader and instrumentation data. Visual representation of the skin health improvements seen across all 3 measurement methodologies are provided for 2 participants (Figures 6 and 7). Representative examples of skin improvements in the MSM group can be seen in Figures 6 and 7. Figure 6 shows global evaluation area for expert graders between baseline and week 16 for participant 1. Figure 7 shows crow’s feet evaluation area for expert graders at all 3 time points for participant 1 and participant 2.
Figure 5. Self-assessment results taken at week 16: Graph demonstrates the percentage of participants responding favorably in each category.
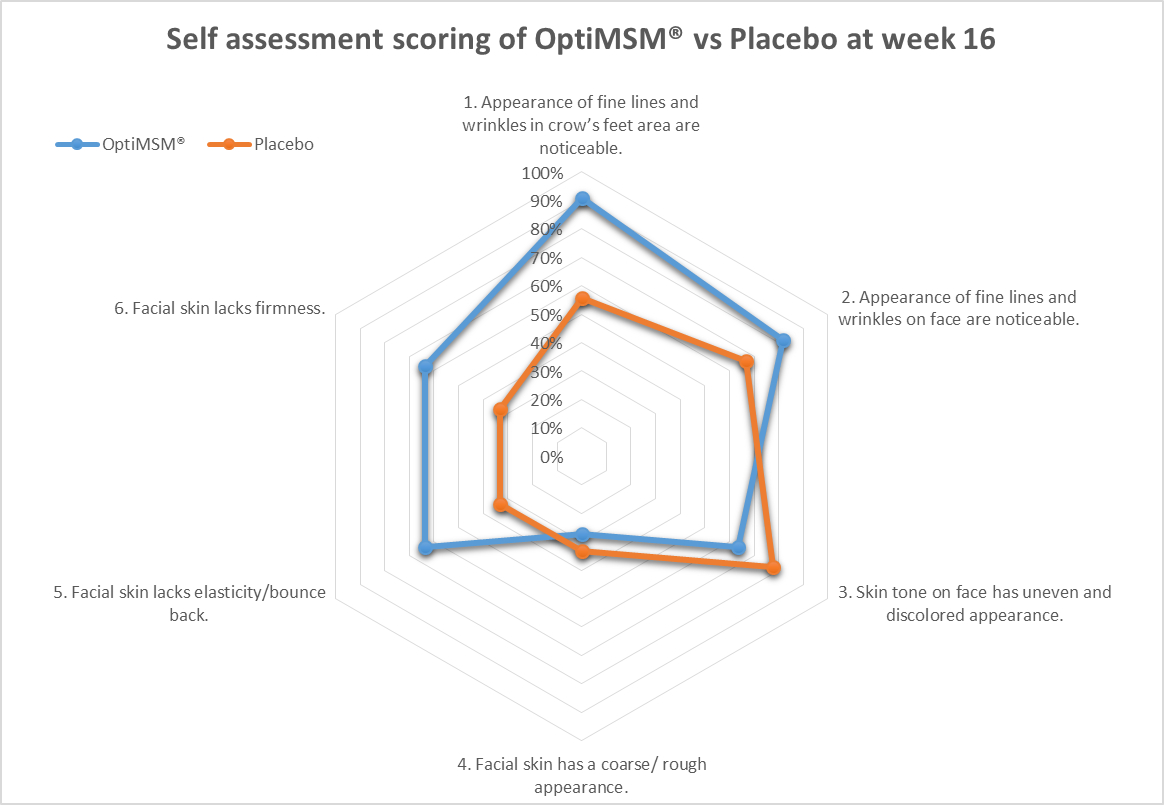
Figure 6. Global facial observation: baseline (left) and week 16 (right) photos of a representative methylsulfonylmethane-treated participant showing improvements in even tone and texture with visibly noticeable reductions in overall facial redness and mottled pigmentation (Participant 2)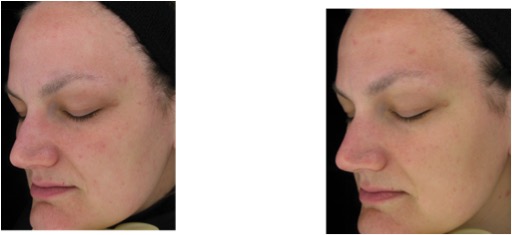
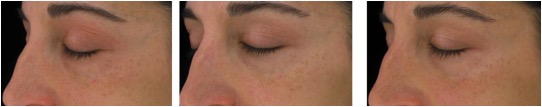
Figure 7. Magnified images of 2 methylsulfonylmethan-treated participants showing improvements from baseline to week 16 of treatment focusing on the crow’s feet area and undereye fine lines and wrinkles as well as overall skin appearance in terms of mottle pigmentation and redness.
Figure 7A. From left to right: baseline, week 8, week 16 (Participant 2)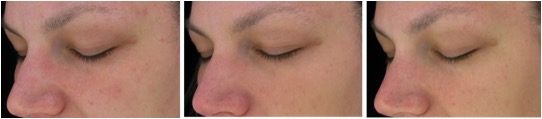
Figure 7B. From left to right: baseline, week 8, week 16 (Participant 1)
Discussion
The process of aging is most often associated with its visible effects on the skin. The skin mirrors the aging process both physiologically and socially.4 Endogenously, the process of aging and its effects on the skin are associated with age-related gene expression, inflammation, hormone levels, and lack of proper nutrition. Exogenously, aging increases exposure to ultraviolet (UV) radiation, toxins, and reactive oxygen species (ROS).28 The skin is affected by both intrinsic and extrinsic factors of aging and manifests largely through the formation of wrinkles, mottled pigmentation, and erythema that can form as a result of both endogenous and exogenous factors.7 Because underlying causes of skin-aging manifest through socially perceptible symptoms, skin health can be assessed through observation, self-perception, and laboratory instruments. The strength of the present study was the assessment of skin health utilizing all 3 methodologies. Expert grading indicated improvements in several markers, especially for the presence of crow’s feet. Participant self-assessment questionnaires indicated overall satisfaction with skin health in both groups. Instrumentation assessments showed a significant improvement over placebo in the number, size, and severity of facial wrinkles, as well as improved barrier function as measured with the vapometer. MSM showed differentiation and improved performance over that of placebo in several areas assessed while placebo did not outperform MSM. Measurable improvements across 3 separate forms of assessment suggest a benefit to skin health from MSM supplementation for the study demographic.
The most noticeable effect throughout all 3 assessments was the reduction in the number, size, and severity of wrinkles. Expert grading showed an improvement in crow’s feet for the MSM group from baseline and also when compared to placebo. Consumer self-perception for wrinkles reported a majority of positive responses to questions about wrinkles. Instrumental analysis utilizing photographic measurements of wrinkles showed those taking MSM had a measurable reduction in the number, size, and severity of facial wrinkles when compared to placebo. The formation of wrinkles results from impaired barrier function, loss of cells, and reduction of underlying extracellular matrix (ECM). MSM may partially improve wrinkles through increased barrier function, as suggested by the vapometer results in this study. Preclinical results also indicate that MSM modifies the genes DSG3 and LCE3D, both of which are associated with barrier function (Table 2). This lends biochemical support to the clinical improvements in barrier function. Other benefits may come from supplying and protecting the ECM and by protecting cells in the dermal and epidermal layers.
A major mechanism of skin aging is the loss or damage of ECM proteins through decreased production or increased damage from endogenous and exogenous sources.29 ECM proteins require disulfide bonds for structural adhesion and formation and require sulfur-containing amino acids (SAA) for protein synthesis.30,31 It has been suggested that MSM sulfur can be incorporated into SAAs, providing necessary building blocks for healthy protein synthesis.18 MSM may also compartmentalize sulfur sources within the body, sparing necessary building blocks (unpublished data). Although MSM’s role in the body’s sulfur pathways may be a contributing factor, it is unlikely to fully account for all observed benefits.
Degradation of the ECM is a major contributor to the aging process.8 MSM may help reduce wrinkles by protecting ECM proteins from damage and degradation by attenuating inflammation and reducing oxidative stress. Previous authors have found that MSM dramatically inhibits the expression of inflammatory cytokines by attenuating NF-κB activation and inhibiting inflammasome activation.32 One study showed MSM inhibits lipopolysaccharide-induced increases in NO and PGE2 production through suppression of inducible nitric oxide synthase and COX-2 expression and that MSM strongly inhibits IL-6 and TNF-α production by suppressing the expression of NF-κB.21 In human chondrocytes, MSM is able to reduce the expression of IL-6 and IL-8 through attenuation of NF-κB and p38 MAPK.33 Several other studies have shown MSM to reduce IL-1, IL-6, and TNF-α, likely through upstream inhibition of NF-κB.34-36 In the skin, inhibition of NF-κB benefits the ECM through reduced expression of inflammatory cytokines and by reduced activation of AP-1. When AP-1 is activated, it stimulates production of collagen-degrading MMPs and it blocks the transforming growth factor-β type II receptors necessary for collagen production.7,37 This may account for the effects of MSM on MMP3 and MMP9 in the preclinical gene expression study (Table 2). The reduction of inflammatory cytokines resulting from the inhibition of NF-κB provides protection for the ECM. TNF-α, IL-1, and IL-6 increase production of ROS and can damage cells responsible for protein production.38,39 Oxidative stress contributes to the degradation of ECM proteins. Increased levels of ROS can result from increased oxidation from exogenous sources, like UV radiation or from endogenous inflammation.38 They can also result from inadequate antioxidative capacity within the body.40 GSH is produced within the body and functions as the primary antioxidant. Several studies have indicated that MSM increases GSH levels and improved overall antioxidant capacity. Several animal studies have shown that treatment with MSM results in increased levels of GSH, SOD, and CAT.20,41,42 In a clinical trial using exercise to induce oxidative stress, MSM was effective in reducing markers of oxidative stress, increase overall levels of GSH, and improve the ratio of reduced to oxidized GSH (GSH/GSSG).43 ECM proteins can be directly damaged by free radicals, altering their structure and function, resulting in skin that is less firm and more prone to wrinkles. ROS also trigger a cascade of downstream inflammatory pathways. Adequate GSH levels mitigate the damage from oxidation and can prevent further inflammation. Initial production of collagen fibers requires sufficient GSH to form crosslinks.30 Homocysteine degrades and inhibits formation of collagen, elastin, and proteoglycans.44 Serum levels of homocysteine are inversely related to levels of GSH, although multiple factors and pathways influence production. People supplementing with MSM have been shown to have decreased levels of homocysteine.14,24 Modulation of NF-κB and/or increased levels of GSH may play a role in MSM’s effect on levels of homocysteine.
Limitations of this study included a small sample size, homogeneity of participants, and seasonal variations that were not controlled for. All participants were women of Caucasian descent within a restricted age range. Homogeneity of participants helped control for variation within a small study population, but results cannot be generalized to the larger population.
Of note, a large placebo effect may have been generated by the strong seasonal variation from baseline to weeks 8 and 16 as the weather moved from winter to spring conditions. Interestingly, the MSM group demonstrated a higher level of melanin production vs the placebo group, though it was not statistically significant. Examining the genes stimulated by MSM, there is a significant increase in bone morphogenic protein 4, which plays a role in promoting pigmentation.45
Conclusion
Under the conditions of this study, use of MSM led to significant improvements in skin’s appearance and condition as evaluated by expert grading, instrumental measures, and participant self-assessment. Although the mechanism of action is not well understood, it is apparent that MSM does work at altering gene expression of key genes that affect moisturization and barrier function, extracellular matrix production, and inflammation control. Inflammation, oxidation, and gene expression in skin cells are highly interconnected, and previous research indicates MSM has a positive effect on all 3, although the root mechanism or mechanisms are not fully understood. The results of this study suggest that the effects of MSM previously characterized through biochemical measurements translate into measurable clinical effects. MSM taken orally may be beneficial for skin health and the reduction of fine lines and wrinkles, though further research is warranted in broader populations.
Conflict of Interest Disclosures
Mr Anthonavage reports personal fees from Bergstrom Nutrition, during the conduct of the study and personal fees from Bergstrom Nutrition, outside the submitted work.
Mr Benjamin reports personal fees from Bergstrom Nutrition - Study Sponser, outside the submitted work. In addition, Benjamin has a patent, US Patent No. 8,217,085 and its foreign counterparts licensed to Biogenic Innovations, a patent, US Patent No. 8,546,373 licensed to Biogenic Innovations, and a patent, US Patent 8,841,100 and its foreign counterparts licensed to Biogenic Innovations.
Mr Withee reports personal fees from Bergstrom Nutrition, during the conduct of the study and outside the submitted work.
Acknowledgements
The authors thank Robert Frumento and Francis Friscia from International Research Services, Inc, for their expertise and guidance regarding the clinical work presented in this manuscript and Ameann DeJohn from Ameann Solutions for her dedication and consultation.








